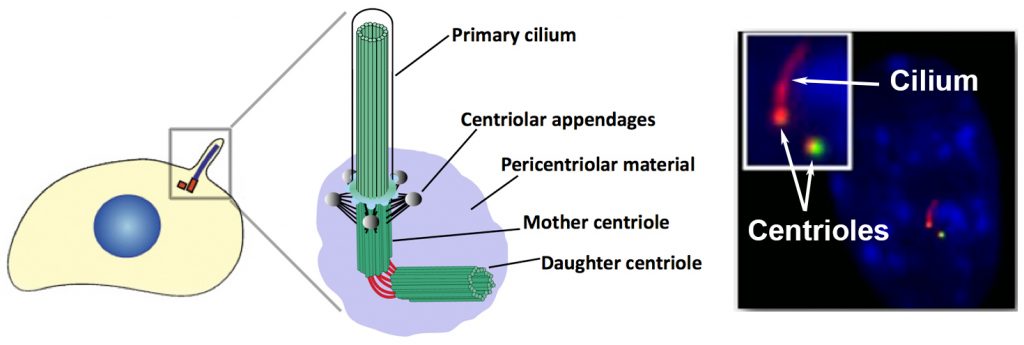
Cilia and Centrosomes: signaling “hubs” in the cell
The proper development of a multicellular organism requires the spatial and temporal organization of signaling pathways that regulate both intra- and inter-cellular communication. As a signaling hub, the centrosome mediates many important aspects of cell physiology, such as orchestrating G1/S transition, entry into mitosis, chromosome segregation, cytokinesis, cell migration and establishment of cell polarity. At the core of the centrosome is a pair of microtubule-based structures called centrioles, which are surrounded by an amorphous mix of proteins termed the pericentriolar matrix. Centrioles play a critical role in cells by serving as basal bodies that nucleate the formation of another important signaling organelle, the primary cilium. Except for a few specialized cell types, almost every cell in the human body contains a primary cilium.
The cilium is a nexus of cell signaling, associated with regulation of the Hedgehog, PDGF, Hippo, Notch, mTOR and Wnt signaling pathways, among others. These signaling molecules are often concentrated within the ciliary space, which is important for efficient pathway function. The primary cilium has been shown to play an essential role in regulating important cellular processes including cell division, differentiation, migration, and polarity.
Main areas of research in the lab:
1 – Cell cycle control of centrosome duplication and ciliogenesis:
Almost all cell types in mammals contain one centrosome, and consequently, a solitary primary cilium. Centrosome and cilium number is maintained by a duplication and segregation mechanism linked to the cell cycle. We are working to determine the molecular mechanisms of centrosome duplication to better understand how it is controlled so that it happens once and only once per cell cycle. This is critical because centrosome duplication errors are a common characteristic of many cancers, and also observed in ciliopathy patients.
2 – Centriole assembly and ciliogenesis in multiciliated cells:
Our lab uses a tracheal epithelial cell (TEC) culture system to identify proteins involved in centriole biogenesis and ciliogenesis. These specialized cells are unique in that, unlike most animal cells that posses one centrosome and one cilium, they generate hundreds of centrioles during differentiation, each one subsequently nucleating a cilium. Roughly ~1000 genes are specifically up-regulated in multiciliated cells during differentiation, including those that encode for structural components of centrioles and cilia, but also the molecular regulators of these processes. This has led to the identification of a number of novel proteins involved in centriole assembly and ciliogenesis. Our goal is to further characterize the ciliogenesis pathway in primary TEC isolated from mouse and human airways:
- Determine signaling pathways that control centriole and cilium assembly
- Identify molecules involved in trafficking of proteins to cilia
- Proteomic analysis of TEC at various stages of differentiation
- Biochemical purification of protein complexes involved in centriole assemble
- Investigate mechanisms that regulate respiratory epithelial cell growth and differentiation, and how those signaling pathways may be altered in diseases affecting the lung.
3 – Consequences of centrosome and cilium dysfunction in human disease:
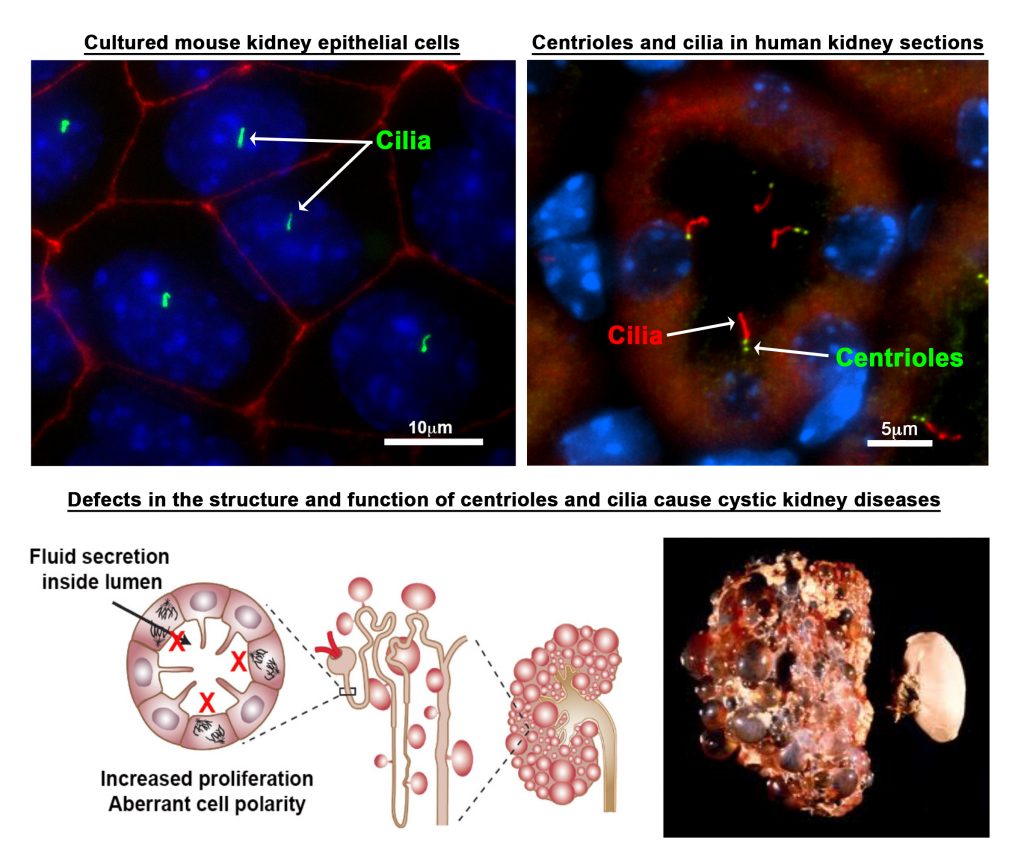
Mutations in centrosomal proteins are known to cause developmental defects that affect multiple organ systems. One organ that is particularly sensitive to these mutations is the kidney. Indeed, mutations that disrupt the structure or function of the centrosome-cilium complex causes cystic kidney disorders such as polycystic kidney disease and nephronophthisis. Thus, one area of investigation involves:
- Determining the consequences of abnormal centrosome biogenesis and its relationship to cystogenesis, using new mouse models of cystic kidney disease
- Characterize changes in centrosome and ciliary-dependent signaling pathways at various stages of PKD